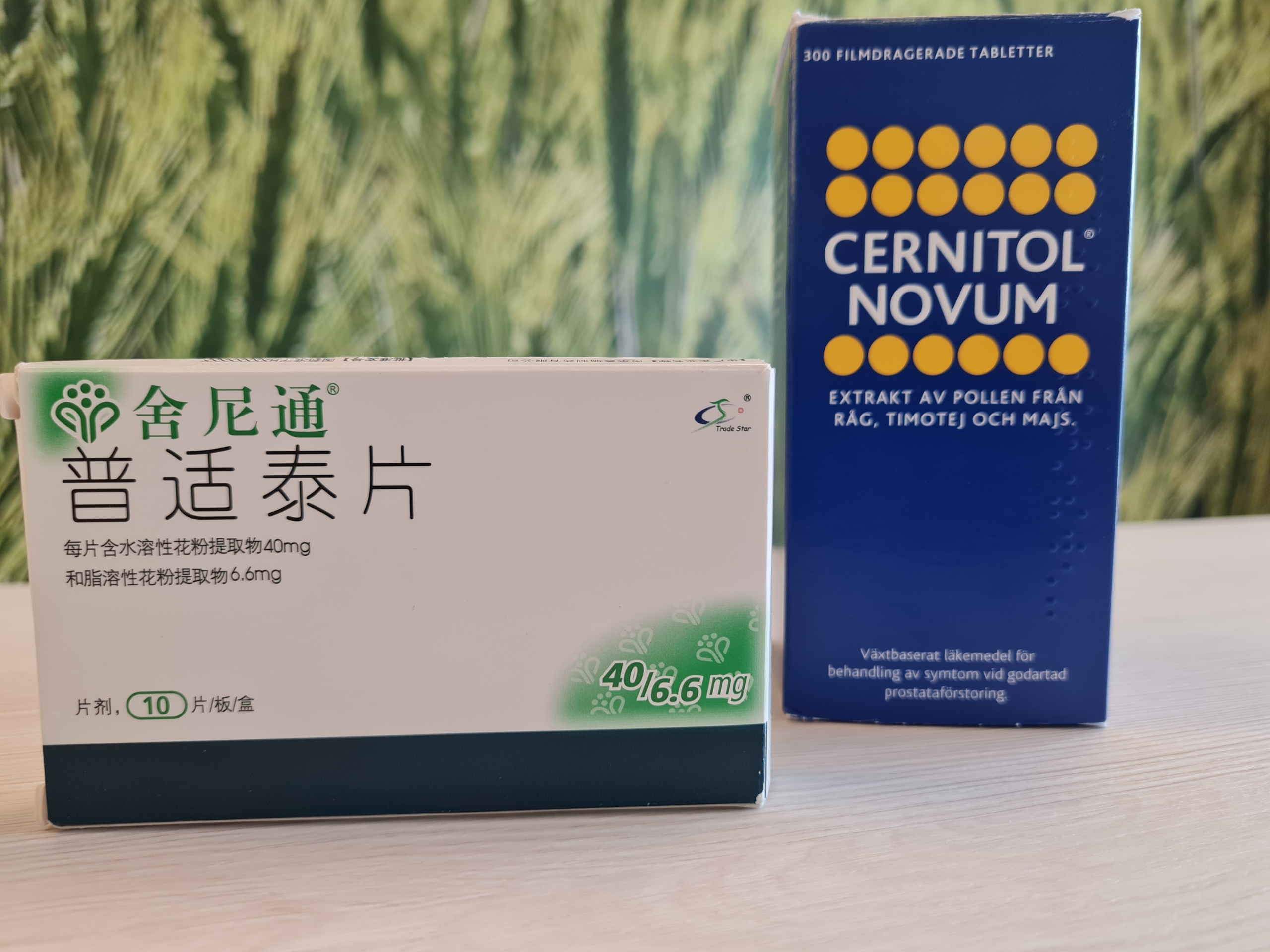
Cernelle enters China.
AB Cernelle, in partnership with MeiRui, a leading Chinese pharmaceutical company specialising in urology, has received approval for Shenitong®, which is an herbal medicinal product for the treatment of benign prostate diseases.
Both companies are now preparing for the market launch of Shenitong®, with the product expected to be available on the Chinese market beginning of 2022.
There are 700 million men in China aged between 15 and 64 years. Around 40 per cent, or 280 million men, are expected to experience symptoms associated with benign prostate conditions. Shenitong® (=Cernitol®Novum) is the only medicinal product that has been approved for treatment of chronic prostatitis in China.
Continued cooperation between Cernelle and MeiRui will now focus on the market launch of Shenitong®, an herbal medicinal product for the treatment of benign prostate conditions, such as chronic prostatitis, pelvic pain syndrome and benign prostate enlargement.
-“This provides Cernelle with a fantastic opportunity, and in the next five years, we expect the company to more than double its production volume and sales value,” says Cernelle CEO, Ken Tinebo.
At present, Cernitol®Novum is manufactured in a volume equivalent to 250 million tablets a year. Intensive work on several projects is under way at the facility outside of Ängelholm to increase production capacity and boost Cernelle into a global leadership in extracting pollen and manufacturing products for medical use, focusing on benign prostate conditions.
-“To achieve this, we need more qualified, dedicated co-workers with the energy and drive to help us in our meaningful work at Cernelle. Recruiting the right people is the key to continued success for the company,” says Ken Tinebo.
Further information: Ken Tinebo, CEO Cernelle, +46 (0)730-877 637, ken.tinebo@cernelle.se
About AB Cernelle
Cernelle is a Swedish pharmaceutical company with research, development, production, and distribution of medicinal products in the urology segment; chronic prostatitis (CP), pelvic pain syndrome (CPPS) and benign prostatic hyperplasia (BPH). Cernelle has been developing herbal pharmaceutical products from high-quality pollen extract since 1953. With over 67 million daily doses per year, Cernilton®/Cernitol® is one of the world’s most widely used medicine to treat benign prostate diseases. The company has 40 employees, located at the facilities outside Ängelholm in Southern Sweden. www.cernelle.com
About MeiRui Pharmaceuticals
A Chinese pharmaceutical company established in 1996 that focuses on the development, manufacture, distribution and sale of urology and allergy medicines in China. MeiRui was formerly owned by Glaxo SmithKline and was sold to Tradestar Medicine & Biotechnology Co Ltd in 2016. Its current owner is an investment company. The company has an annual turnover of approximately SEK 3,000 million.